Overview
ChemSee offers detectors designed for easy, home-based detection of cyanide in food, drinks and other liquids. Simple, color-based test results are obtained in 3 seconds to 3 minutes.
Detectors use detection technology validated by the U.S. Department of Defense and North Carolina State University. More information can be found on our commercial section here: https://www.chemsee.com/commercial/food-poisons/resources/validation/
Available Products
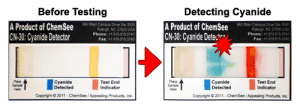
Cyanide Detectors
- Gives Simple, Color-based Results
- Detects Cyanide in 5 Seconds to 8 Minutes
- Includes Built-In Test End Indicator
- Small, Lightweight Detector can be Easily Concealed
- Provided with Full Color Instructions
- Uses Independently Validated Technology

Decontamination of Cyanide
- Rapidly Neutralizes Cyanide Poisons,
- Does not cause Environmental Damage,
- Non-Corrosive and Non-Toxic,
- Neutralized Cyanide can be Disposed of in Normal Waste-Water,
- Low-Cost,
- Can Be Used Even By Untrained People Safely To Rapidly Neutralize Cyanide Residues, and,
More Information
On the Feasibility of Poisoning with Cyanides and How to Prevent it.
By: Dr. A. J. Attar
Appealing Products Inc.
6003 Chapel Hill Rd. Ste #177
Raleigh, NC 27607 USA.
Phone: 001-919-916-4908
Fax: 001-919-916-4910
E mail: ajattar@appealingproducts.com
1. Why Cyanides are the Ideal Poison for Want-To-be Poisoners?
Cyanide offers Want-To-Be Poisoners unique combination of properties that makes it an ideal poison for professionals as well as for amateurs. The main ones are:
A. Cyanides are extremely toxic and only a small amount of material is sufficient to kill a person.
For example, the probability that a person who weighs 160 lbs, (72.64 Kg), who ingests 0.3632 grams potassium cyanide will die within three days is 50 %, and if he ingests 0.55 grams, the probability is over 90 %. To put these numbers in prospective, a teaspoon of salt contains 7.2 grams and a tablespoon contains 23.7 grams. Thus, 90 percent of 13 people, about 12, who weigh 160 lbs can be killed using a SINGLE teaspoon of potassium cyanide. A single tablespoon of potassium cyanide will kill 90 % of 43 people, i. e. about 39. In reality, death due to cyanide poisoning will occur in much shorter time than three days, more likely in 2-6 hours.
To put this in prospective, 0.36 grams of potassium cyanide, a white crystalline material that looks like table salt or coarse sugar, occupies a smaller volume then the volume of salt that most people put on an order of French Fries. It can also be mixed with ordinary sugar or placed in a packet of artificial sweetener. If the mixing ratio is say 1 cyanide to 9 sugar, one or two spoons will contain enough cyanide to kill the user! Crystalline potassium cyanide will not be distinguishable by the eyes from ordinary salt or sugar and bot will readily dissolve in water, tea or coffee.
Added Info on Toxicity Measurements.
The toxicity of materials is measured by a term called the LD50. The LD50 is the quantity of toxic material, expressed in grams per Kg body weight of the person being poisoned, that will kill 50 % of the population in three days. For example, the LD50 for Potassium Cyanide, KCN, is 5 mg/Kg and for sodium cyanide, NaCN, it is 6.4 mg/kg.
The values of the LD50 are determined by feeding the poison to many animals of the same kind, say rats, and calculating the LD50 based on their weight. This approach uses several basic assumptions which are not strictly correct. These assumptions are that:
- The toxic effect is related linearly through a relatively large range of body weights, to the body weight of the victim, and,
- The mechanism of toxicity is the same for “similar animals”, i. e. one may conduct toxicity tests on say rats or mice and apply the LD50 data to human, since both are mammals.
B. Cyanides toxicity shows in relatively very short time.
Inorganic cyanides, the most available ones, are very toxic when ingested but their solution in water is even more toxic. Potassium, sodium and many of the other inorganic cyanides are water soluble. Therefore, when ingested, they quickly form solutions in the stomach which rapidly enters the blood and circulate through every part of the body. The toxicity is somewhat reduced if alcohol and sugar are present simultaneously with the cyanide. The reason is that the cyanide ion can react with the sugars to form amygdalin. This compound is not very stable and decomposes in water to reform cyanides and sugar. (Hydrolysis). Anecdotally, when attempts were made to poison the Russian Healer/Advisor to the Czar, Rasputin, by putting cyanide in his red wine and cake, he did not die. The exact confirmed evidences are sketchy, but one possibility is that the effective amount of cyanide ingested was reduced due to its reaction with the sugars in the wine to form the less toxic amygdalin.
Cyanides react with acids to form the volatile and deadly hydrocyanic acid, HCN. A few cases were documented where people died after breathing HCN vapors released where attempts were made to clean cyanide-containing vessels with acids. A laboratory cleaning lady died a few years ago breathing HCN vapors released from a sink after pouring into it a cleaning acid. Previous worker poured in it cyanide.
The action of cyanides and hydrocyanic acid on living animals, in particular on mammals, is attributed by and large to their irreversible reaction with the iron ions in the blood. This effects the assimilation and transportation of oxygen in the blood to the various parts of the body, which has dire effect on the functioning. Other mechanisms were proposed and are believed to play a strong role in cyanide toxicity, however, the rapid toxicity is believed to be due to the cyanides interrupting the absorption and assimilation of oxygen. Since the availability of oxygen is so critical to adequate functioning of the brain and muscles, death due to cyanide poisoning occurs in a relatively short time. Other metallic ions also react with cyanide ions.
Although the toxicity of cyanides is compared with that of other poisons using the LD50, in effect, the toxic effect is much more rapid than three days. Moreover, if the victim was reasonably healthy and did not die within a reactively short time, there is a reasonable chance that he will recover. Several antidotes have been used to overcome the effect of cyanide, but their efficacy is limited. Moreover, it is frequently too late to administer the antidote by the time it is realized that the person was poisoned with cyanide.
Cyanide poisoning may be recognized by a smell similar to that of almonds emanating from the victim, his vomit or feces. This smell is somewhat similar to that of Amaretto. The blood of victims of cyanide poisoning appears somewhat bluish, due to the formation of iron complexes with the cyanides. (Prussian Blue).
C. Cyanides are readily available from many different sources.
Cyanides are used in many industries and thus are available to potential poisoners. The main industries that use cyanides are:
- The mining of gold from low-grade ores.
- The mining of silver from low grade silver ores.
- The electroplating industry.
- The pesticides industry: Some countries such as New Zeeland permit using cyanide-containing pesticides in various tasks.
- The precious metals recovery industry, e. g. the recovery of silver in photography.
- Chemical laboratories, pharmaceutical manufacturers etc.
The commerce of use of cyanides in most countries is tightly controlled and exporting and importing large quantities of cyanides is a very regulated commerce. In some countries a chain of custody procedure is required to track the use of cyanide. However, these procedures are not easily regulated since many applications result in the consumption of cyanides. However, since only a very small amount of cyanides is sufficient to kill a person, obtaining enough material to cause harm is not that difficult.
D. Cyanides can be made by amateurs from readily available sources.
Many readily-available materials release hydrocyanic acid, HCN, when heated. (This process is called pyrolysis). The gaseous HCN can be recovered if the gas is passed through a solution containing a base such as sodium carbonate, (Available in hardware stores and food stores), baking soda, sodium hydroxide, borax and to a lesser extent even ammonia. This procedure produces impure solution of cyanide which often can be used to poison as is, or purified by a very simple process to produce almost pure sodium cyanide.
Some of the materials which are widely available and which can be readily decomposed by heat, pyrolysed, to produce hydrocyanic acid are:
- Shells of almonds, in particular fresh and somewhat green shells or even leafs of bitter almonds.
- Pitts of fruits such as peaches, plums, cherries, apricots, mangoes, etc.
- The roots of cassava (Manioc), in particular if they are not ripe. The bitter cassava produces more hydrocyanic acid and many people died after eating improperly prepared cassava.
- Plastic parts made of Polyurethane, including polyurethane-based paint.
- Plastics such as polyacrylonitril and cyanoacrylates. (“Superglue”).
- Lignite and low rank coal. (All coals release HCN but lignite releases more than bituminous).
Larger yields of cyanide are obtained if fresh air is not available to contact the heated material during the pyrolysis. Passing a small stream of nitrogen or helium over the heated material and then into the basic solution increases the yield of hydrocyanic acid. Although the yield of cyanides in these processes is not large, enough cyanide can be recovered to poison a person since the quantity needed is very small. Some victims who die due to smoke inhalation and fire actually die from breathing excess HCN. Most states do not allow using polyurethane mattresses, in particular in prisons, because the gases emitted upon their combustion in limited air were the cause of death of some inmates.
2. Symptoms of Cyanide Poisoning.
The main symptoms of acute poisoning by cyanides are due to the interference of cyanide in the assimilation and distribution of oxygen in the body. Whether the cyanide source is HCN or ingestion of food containing a water soluble cyanide such as potassium or sodium salt, the poisoning mechanism is the same. The cyanide ions react irreversibly with the iron, in particular with the iron in the cytochrome c oxidase and hemoglobin. Since cyanides interfere with the absorption of oxygen and thus with the production of energy in the body, an increased exposure to cyanide will gradually show as headache, nausea, confusion, weakness, fatigue, loss of coordination, hyperventilation, cardiac arrhythmia, bradycardia, loss of consciousness and coma. Death typically occurs due to problems with the nervous system or the heart.
Post mortem analysis of blood of poisoned people shows that concentrations of about 3 micrograms cyanide per ml were sufficient to kill the person. Incorporation of cyanide, mainly HCN, via the breathing, is a lot more rapid than via ingestion. A person will die instantly if the HCN concentration is above 270 ppm, (Parts per million), and after 10 and 30 minutes respectively if the HCN concentrations are respectively 180 and 130 ppm.
Since cyanides effect the function of muscles and produce confusion, many victims die because they lose their coordination and ability to escape to areas with clean air. This happens sometimes when people are trapped in a burning house and the air contains cyanide.
3. Detecting Cyanides.
If it is suspected that a victim was poisoned by ingesting cyanide, it imperative that it will be determined QUICKLY since the poisoning effect of cyanide is very quick. To this end, Appealing products Inc., API, developed a simple, low cost kit for detecting poisons, including traces of cyanides, in 3-8 seconds in liquids such as water and in 2-8 minutes in any food. These detectors allow emergency personnel to react timely and save the victim. http://chemsee.com/poison-detection/products/. The KT-06 kit detects cyanides and up to 30 other poisons. http://chemsee.com/poison-detection/products/api-poison-detection-kit/. The STPD-06 detects the same poisons but has a much shorter shelve life. http://chemsee.com/poison-detection/products/stpd-poison-detection-kit/. The CA-61K kit detects only cyanides, azides, sulfides and chromates. These kits were tested by the US Department of Defense who validated their performance http://www.tswg.gov/subgroups/cbrnc/detection/detection-of-toxic-adulterants-in-food.html as well as by the Food Science Department of NC State University, http://appealingproducts.com/secure/ncsuval/.
Cyanides may be detected using conventional laboratory techniques and instruments. Unfortunately, these techniques require expensive and sophisticated instrumentation and highly trained personnel and the results are NOT obtained immediately. Since in real emergency situations such amenities may not be available, and a timely response is critical to save a person live, the value of the laboratory services is mainly in post mortem investigations.
4. Antidotes to Cyanides.
Since the poisoning mechanism of cyanides is based on the reaction of the free cyanide ion with metals, mainly iron and thus preventing the proper absorption and assimilation of oxygen, antidotes to cyanide poisoning utilize injecting relatively large amounts of metallic ions which rapidly and irreversibly react with the cyanide ion. This process depletes the concentration of the cyanide ions in the blood and thus reduces the amount of cyanide available for reaction with the iron and deactivating it. The most important qualities of a good antidote for cyanide poisoning are:
- It has to react with the cyanide ion very rapidly and irreversibly.
- It has to be relatively non-toxic and its reaction product with the cyanide has to be non-toxic and easily eliminated from the body.
- It has to be compatible with the biochemical in the blood and relatively inert to them, and,
- It has to have a long shelve life so that it can be transported and stored for emergency use.
One of the antidotes used today is hydroxocobalamin, a naturally available form of vitamin B12. This compound reacts with cyanide to form cyanocobalamin, which is eliminated safely by the kidneys. The cyanide reacts with the cobalt in the hydroxocobalamin and thus consumed and becomes unavailable to react with the iron. An antidote kit based on this chemistry is sold under the brand name Cyanokit and was approved by the FDA in 2006.
The most important mechanism which eliminates cyanide from the body involves the enzymatic conversion of the cyanide to the relatively non-toxic thiocyanate ion. (CNS-1). This process is done by the mitochondrial enzyme rhodanese. Taking some sodium thiosulfate accelerates the detoxification by providing a readily-available source of sulfur to the rhodanese. Unfortunately, the thiosulfate depletes some of the minerals in the body.
Inhalation of nitrites assists in overcoming breathing problems due to cyanide. Traditionally, many emergency kits used to contain ampules of amyl nitrite to help revive people who ingested cyanides or breathed carbon monoxide or cyanogen or hydrocyanic acid. Since the amyl nitrite ampoules were diverted to other applications, many kits do not contain them any longer.
U.S. Department of Defense Validation
The KT-06 was developed for the U.S. Department of Defense through TSWG, and validated by them independently.
“Current risk assessments combined with historical evidence indicate that soft targets such as the food and water supply may represent a point of entry for terrorist attacks. Appealing Products, Inc. has developed a self-contained, deployable food security test kit that can detect sublethal concentrations of toxic chemicals (cyanides, azides, and sulfides) and metallic poisons (arsenic, mercury, lead, cadmium, thallium, and chromium) in a wide range of food matrices.”
You may read more on the TSWG website, found at: https://www.cttso.gov/sites/default/files/reviewbook/2006_Review_PDF_Linked.pdf.
North Carolina State University Validation
Additionally, an Independent Technical Validation of our Food Poison Detection Kit has just been completed by Professor Jonathan Allen of the Department of Food, Bioprocessing and Nutrition Sciences at North Carolina State University. Professor Allen in conclusion wrote: “Our overall opinion is that API’s detectors for poison in food readily detect dangerous levels of poison yet they respond very rapidly and are easy to use even by a layman”.
The full NC State University Report is available online at: http://appealingproducts.com/secure/ncsuval/.